Synopsis
Spec references J248: C2.2h J250: C2.2h. Professor Jim Al-Khalili explains how chemical properties are determined by electronic structure using the analogy of a tower block. (If the upper floor is full, any additional electrons added will have to look for an apartment on the next floor up).
- Programme: Atom
- Episode: The Clash of the Titans
- Channel: BBC Four
- Broadcast year: 2007
-
Chemistry (J248): C2.2h
Combined Science (J250): C2.2h
Licence: ERA Licence required
UK only
Staff and students of licensed education establishments only
Cannot be adapted
Add Notes
More clips from Atom

Heisenberg's Uncertainty Principle | Atom
Heisenberg's Uncertainty Principle | Atom
Jim Al-Khalili explains Heisenberg's famous Uncertainty Principle that explains why an atom can't be visualised.

How Rutherford Discovered the Nucleus | Atom
How Rutherford Discovered the Nucleus | Atom
Spec references J248: C1.2a J250: C1.2a. Detailed desciption of how Rutherford's alpha particle scattering experiment was develo...

The Clash of the Titans | Atom
The Clash of the Titans | Atom
Nuclear physicist Jim Al-Khalili tells the story of the discovery that everything is made of atoms, and of the minds behind the scientific bre...

The quantum jump | Atom
The quantum jump | Atom
Jim Al-Khalili attempts to explain one of the most difficult scientific concepts, but one that underpins everything.

Heisenberg's Uncertainty Principle | Atom
Heisenberg's Uncertainty Principle | Atom
Jim Al-Khalili explains Heisenberg's famous Uncertainty Principle that explains why an atom can't be visualised.

How Rutherford Discovered the Nucleus | Atom
How Rutherford Discovered the Nucleus | Atom
Spec references J248: C1.2a J250: C1.2a. Detailed desciption of how Rutherford's alpha particle scattering experiment was develo...

The Clash of the Titans | Atom
The Clash of the Titans | Atom
Nuclear physicist Jim Al-Khalili tells the story of the discovery that everything is made of atoms, and of the minds behind the scientific bre...

The quantum jump | Atom
The quantum jump | Atom
Jim Al-Khalili attempts to explain one of the most difficult scientific concepts, but one that underpins everything.
More resources about Particle Model of Matter

03: Off the Scale | Richard Hammond's Invisible Worlds
03: Off the Scale | Richard Hammond's Invisible Worlds
Richard Hammond explores the miniature universe around us, from microscopic changes to ice crystals that can trigger a...

Atmospheric pressure | Short Circuit
Atmospheric pressure | Short Circuit
. Atmospheric pressure is related to the weight of the air above you.

Dissolved gases under pressure | Short Circuit
Dissolved gases under pressure | Short Circuit
. Spec references: J249: P1.3c, P1.3d, P1.3f Analogy between dissolved gases in the blood and fizzy drinks.
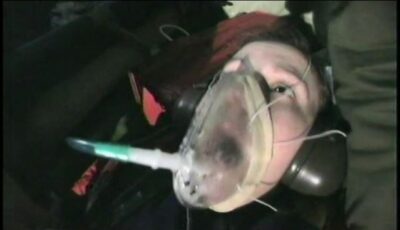
Height and pressure | Short Circuit
Height and pressure | Short Circuit
Effect of height above the surface of the Earth on pressure - diving patient on a helicopter flight.

Heisenberg's Uncertainty Principle | Atom
Heisenberg's Uncertainty Principle | Atom
Jim Al-Khalili explains Heisenberg's famous Uncertainty Principle that explains why an atom can't be visualised.

How Rutherford Discovered the Nucleus | Atom
How Rutherford Discovered the Nucleus | Atom
Spec references J248: C1.2a J250: C1.2a. Detailed desciption of how Rutherford's alpha particle scattering experiment was develo...
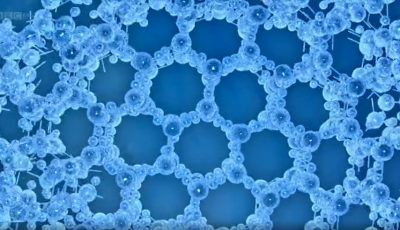
Ice crystal properties | The Secret Life of Ice
Ice crystal properties | The Secret Life of Ice
Dr Gabrielle Walker and Dr Andrea Sella investigate the properties of ice crystals, to explain why ice floats.

Pressure | Short Circuit
Pressure | Short Circuit
Bringing chemistry, physics and biology to life using real-life examples, 3D graphics and practical experiments.

Pressure and water depth | Short Circuit
Pressure and water depth | Short Circuit
Spec references J249: P1.3c, P1.3d, P1.3f. Effect of water depth on an enclosed volume of air - scuba diver.

Pressure on ice | The Secret Life of Ice
Pressure on ice | The Secret Life of Ice
Dr Gabrielle Walker and Dr Andrea Sella investigate what happens when pressure is applied to ice and how this allows us to ice skate.

The Clash of the Titans | Atom
The Clash of the Titans | Atom
Nuclear physicist Jim Al-Khalili tells the story of the discovery that everything is made of atoms, and of the minds behind the scientific bre...

The decompression chamber | Short Circuit
The decompression chamber | Short Circuit
Spec references J249: P1.3c, P1.3d, P1.3f. How a decompression chamber works - animation and analogy with diving context .

The effect of pressure on gases | Short Circuit
The effect of pressure on gases | Short Circuit
Spec references J249: P1.3c, P1.3d, P1.3f. The effect of pressure on bubble size - animation.

The quantum jump | Atom
The quantum jump | Atom
Jim Al-Khalili attempts to explain one of the most difficult scientific concepts, but one that underpins everything.
What is the bends? | Short Circuit
What is the bends? | Short Circuit
. How bubbles in the blood cause the bends.

Physical Processes 3: Temperature and Heat | Scientific Eye
Physical Processes 3: Temperature and Heat | Scientific Eye
The show delves into the nature of heat as a form of energy transfer,
More from Jim Al-Khalili

2: The Order of the Elements | Chemistry: A Volatile History
2: The Order of the Elements | Chemistry: A Volatile History
Professor Jim Al-Khalili looks at how the early scientists' bid to decode the order of the elements was driven b...

Einstein and the beetle | Gravity and Me: The Force that Shapes our Lives
Einstein and the beetle | Gravity and Me: The Force that Shapes our Lives
Professor Jim Al-Khalili explains Einstein's analogy of gravity that he told his son to explain his...

Prof Jim Al Khalili goes inside Calder Hall for his first look inside the core of a nuclear reactor | Britain's Nuclear Secrets: Inside Sellafield
Prof Jim Al Khalili goes inside Calder Hall for his first look inside the core of a nuclear reactor | Britain's Nuclear Secrets: Inside Sellafield
Nuclear physicist Professo...

Sarah-Jayne Blakemore on teenage brains | The Life Scientific
Sarah-Jayne Blakemore on teenage brains | The Life Scientific
Professor Sarah-Jayne Blakemore talks to Jim Al-Khalili about her research on the developing teenage brain.
Semiconductors and WWII | Shock and Awe: The Story of Electricity
Semiconductors and WWII | Shock and Awe: The Story of Electricity
Jim Al-Khalili explains the importance of semiconductors, particularly with regard to fighting WWII.

Fukushima: Is Nuclear Power Safe? | Horizon
Fukushima: Is Nuclear Power Safe? | Horizon
Six months after the explosions at the Fukushima nuclear plant in Japan, Professor Jim Al-Khalili sets out to discover whether nu...

Al-Khwarizmi's digit and numerical system | Science and Islam
Al-Khwarizmi's digit and numerical system | Science and Islam
Jim Al-Khalili explains how our numerical system spread to Europe through the work of the Arabic mathematician ...

The big bang and the creation of hydrogen and helium | Atom
The big bang and the creation of hydrogen and helium | Atom
George Gamow believed that helium existed in the universe before the sun and the stars were formed.

Electrolysis - Discovery of Potassium | Curriculum Bites
Electrolysis - Discovery of Potassium | Curriculum Bites
Spec references J248: C3.4b J250: C3.4b. Professor Jim Al-Khalili recreates the electrolysis experiment that lead to...
