Atoms, elements and compounds

How are elements made? | Wonders of the Universe
How are elements made? | Wonders of the Universe
Brian Cox explains what elements are and how they are formed, including the importance of carbon.

Types of Compounds | Curriculum Bites
Types of Compounds | Curriculum Bites
Spec references J248: C2.2h J250: C2.2h. An introduction to how elements react to form ionic and covalent compounds, with reference to ...

Uses of Transition Metals | Short Circuit
Uses of Transition Metals | Short Circuit
An extra-curricular link is made to transition metals, with an explanation of how metal oxides are used to produce stained glass. T...

Ammonia | Short Circuit
Ammonia | Short Circuit
Bringing chemistry, physics and biology to life using real-life examples, 3D graphics and practical experiments.

Aluminium | Short Circuit
Aluminium | Short Circuit
Bringing chemistry, physics and biology to life using real-life examples, 3D graphics and practical experiments.

Elements | Curriculum Bites
Elements | Curriculum Bites
Schools science programme where topics in the double science curriculum are broken down into small chunks.
The Periodic Table trends and history
1: Discovering the Elements | Chemistry: A Volatile History
1: Discovering the Elements | Chemistry: A Volatile History
The story of how the elements were discovered and mapped begins with the alchemists who questioned that the world...

2: The Order of the Elements | Chemistry: A Volatile History
2: The Order of the Elements | Chemistry: A Volatile History
Professor Jim Al-Khalili looks at how the early scientists' bid to decode the order of the elements was driven b...

Helium | Chemistry: A Volatile History
Helium | Chemistry: A Volatile History
Norman Lockyer recorded a new yellow line by observing the sun through London smog and, assuming the new element to be a metal, named ...

Gallium | Chemistry: A Volatile History
Gallium | Chemistry: A Volatile History
Gallium is in a liquid state over the widest range of temperatures than any other element.

Periodic Table Overview | Chemistry: A Volatile History
Periodic Table Overview | Chemistry: A Volatile History
Spec references J248: C2.2i J250: C2.2i. A quick recap of how the periodic table is arranged including groups, period...
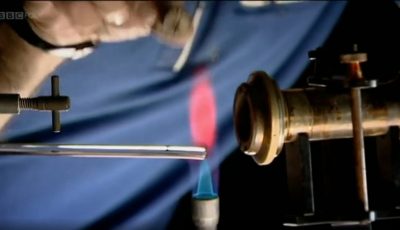
Bunsen Burner | Chemistry: A Volatile History
Bunsen Burner | Chemistry: A Volatile History
Robert Bunsen was a German chemist responsible for the invention of the Bunsen Burner.
Silicon | Chemistry: A Volatile History
Silicon | Chemistry: A Volatile History
Silicon was discovered by Jöns Jacob Berzelius, a Swedish chemist, in 1824 by heating chips of potassium.

Properties of Group 1 Metals | Chemistry: A Volatile History
Properties of Group 1 Metals | Chemistry: A Volatile History
Spec references J248: C4.1a. Demonstration of reactions of lithium, sodium and potassium with water (Contains hi...

Mendelleev's Periodic Table | Chemistry: A Volatile History
Mendelleev's Periodic Table | Chemistry: A Volatile History
Spec references J248: C2.2i J250: C2.2i. An account of how Mendeleev arranged the elements by atomic mass to crea...
Properties of Potassium | Chemistry: A Volatile History
Properties of Potassium | Chemistry: A Volatile History
Spec references J248: C4.1a J250: C4.1a. Professor Jim Al-Khalili describes properties of potassium and some of its u...

Lavoisier's Elements | Chemistry: A Volatile History
Lavoisier's Elements | Chemistry: A Volatile History
Lavoisier was the first scientist to define what an element was.

Hydrogen | Chemistry: A Volatile History
Hydrogen | Chemistry: A Volatile History
Cavendish accidentally discovered hydrogen, the first elemental gas, when experimenting with air.

Oxygen | Chemistry: A Volatile History
Oxygen | Chemistry: A Volatile History
The story of how Joseph Priestley discovered oxygen.

The discovery of phosphorus | Chemistry: A Volatile History
The discovery of phosphorus | Chemistry: A Volatile History
Phosphorus was discovered when Brandt was attempting to isolate gold from his urine.

Mercury | Chemistry: A Volatile History
Mercury | Chemistry: A Volatile History
Mercury is fourteen times heavier than water, and so dense even a steel bolt will float in it.

Arrangement of the Periodic Table | Curriculum Bites
Arrangement of the Periodic Table | Curriculum Bites
Spec references J248: C2.2a, C2.2b, C2.2c J250: C2.2a, C2.2b, C2.2c. This video explains the arrangement of the periodic...



